Replace: After this story was printed, an up to date draft agenda for the ACIP assembly was posted. Based on the schedule, the committee will not contemplate the RSV vaccine, however it would vote on the three different pictures described on this story: COVID-19, hepatitis B and MMRV.
Subsequent week, the panel of high advisers who suggest how vaccines are utilized in the USA will meet to evaluate jabs that shield towards COVID-19, hepatitis B and different ailments. The assembly — the committee’s second since US well being secretary and anti-vaccine advocate Robert F. Kennedy Jr abruptly fired all of its earlier 17 members and welcomed 7 new ones — has raised eyebrows amongst public-health specialists, on condition that the protection and efficacy of among the vaccines on the agenda have been effectively established for years.
“I’m very involved, given the signalling from the members of this newly reconstituted, hand-picked [committee] that they’re going to choose targets for additional restriction,” says Andrew Pavia, a doctor with the Infectious Ailments Society of America, a medical affiliation primarily based in Arlington, Virginia. At their final assembly, the advisers — a number of of whom have publicly expressed anti-vaccine views — voted to finish using the preservative thimerosal in influenza vaccines, regardless of proof that it’s secure on the doses present in jabs.
On supporting science journalism
For those who’re having fun with this text, contemplate supporting our award-winning journalism by subscribing. By buying a subscription you might be serving to to make sure the way forward for impactful tales in regards to the discoveries and concepts shaping our world at present.
It’s unclear precisely what the Advisory Committee on Immunization Practices (ACIP) will vote on at subsequent week’s assembly, to be held on 18 and 19 September, as a result of the agenda lacks element, rising researchers’ concern. “It’s very uncommon for ACIP to place issues on the agenda with solely a month’s discover and never talk about who’s going to be presenting knowledge, what knowledge goes to be checked out, and what, if any, questions would possibly come up for a vote,” Pavia says.
The US Division of Well being and Human Companies, which is run by Kennedy and has authority over the ACIP, didn’t reply to Nature’s request for remark.
Nature spoke to public-health specialists in regards to the vaccines up for dialogue, what the committee would possibly vote on and the info it must be contemplating.
Scrutiny for COVID-19 jabs
Final month, the US Meals and Drug Administration (FDA) authorized up to date COVID-19 vaccines, nevertheless it imposed limitations on who can get the pictures. Whereas the vaccines had been beforehand licensed for all individuals aged 6 months and older in the USA, now they’re authorized just for these older than 65 years, in addition to individuals with well being situations that put them at excessive threat of extreme illness.
The FDA is liable for authorizing new vaccines, whereas the ACIP, overseen by the US Facilities for Illness Management and Prevention (CDC), advises on who ought to obtain them. These suggestions, that are sometimes adopted by the CDC as official coverage, assist to tell which vaccines are coated by medical insurance.
“I assume ACIP may find yourself mirroring what the FDA has really helpful” for COVID-19 pictures, says Adam Ratner, a paediatrician on the American Academy of Pediatrics (AAP), a company primarily based in Itasca, Illinois.
In a press release following the FDA’s resolution on 27 August, AAP president Susan Kressly known as the transfer “deeply troubling”, particularly for kids, whose security Kennedy has centered on when questioning vaccines. The earlier week, the AAP issued its personal suggestion that each one kids 6 months and older must be vaccinated and, specifically, children aged 6–23 months outdated who’re at excessive threat of extreme COVID-19.
Based on knowledge from the 2022–23 chilly and flu season, mRNA-based COVID-19 vaccines had been between 46% and 70% efficient at stopping COVID-19-related emergencies for kids aged 6 months as much as 5 years throughout a 2-month window after the second or third dose. (Flu vaccines, that are additionally up to date seasonally, are sometimes 40–60% efficient.) And such pictures elicited no security considerations in that very same age group.
Nonetheless, The Washington Put up is reporting that Trump well being officers would possibly hyperlink COVID-19 vaccines with 25 paediatric deaths reported to a vaccine security database at subsequent week’s assembly. Anybody can submit studies to the database, however claims should be investigated to verify a hyperlink.
Hepatitis B pictures not for newborns?
Anti-vaccine advocates have been questioning whether or not a hepatitis B vaccine must be given to newborns — the present apply in the USA. As an alternative, they’re suggesting that jabs be administered solely to individuals with sure high-risk behaviours, together with unprotected intercourse and drug use, Pavia says. That’s an issue, he provides, as a result of “the vast majority of hepatitis B worldwide is transmitted from mom to toddler”.
Hepatitis B is a liver illness attributable to a virus that spreads by contact with contaminated blood and different physique fluids. Based on the AAP, infants contaminated with hepatitis B of their first yr of life have a 90% probability of creating power illness, and one-quarter of these with power hepatitis B die from it.
Nature; Supply: CDC/Nationwide Notifiable Ailments Surveillance System (knowledge)
Since the USA started recommending hepatitis B vaccines for infants within the Nineteen Nineties, circumstances have dropped considerably (see ‘Generational decline in hepatitis B’). Administering “earlier is at all times higher with an incurable virus”, says Peter Chin-Hong, an infectious-disease doctor on the College of California, San Francisco.
Some assume that the ACIP panel will suggest eliminating hepatitis B vaccines for infants, as an alternative counting on screening pregnant individuals for the illness — and vaccinating solely the infants of those that take a look at optimistic. However “there will be false negatives”, Ratner says. And “there will be people who find themselves screened, after which the end result just isn’t communicated effectively” so motion just isn’t taken. Giving the vaccine at start is subsequently probably the most strong safety, he provides. Research have demonstrated the protection of vaccinating newborns for hepatitis B.
Stricter recommendation for measles jabs?
In 2008, the US vaccine security system recognized an elevated threat of fever-induced seizures in kids between one and two years of age who acquired the measles, mumps, rubella and varicella (MMRV) vaccine (varicella is often referred to as chickenpox). That statement led the CDC to suggest that kids in that age group obtain separate pictures for his or her first dose — one for measles, mumps and rubella (MMR) adopted by one for varicella — except a father or mother expressed a desire for the one MMRV shot. For the second dose, sometimes given to kids 4–6 years of age, the company really helpful the mix MMRV jab, as a result of there weren’t security considerations for that age group.
Over the last ACIP assembly, chair Martin Kulldorff introduced the info from 2008 and proposed that the MMRV vaccine by no means be administered to kids underneath the age of 4 years. That is in keeping with what the CDC already recommends, so it’s doable that Kulldorff merely desires to strengthen the language of the steerage. Nonetheless, strongly opposing the MMRV jab may have its downsides: the mix shot makes it simpler for individuals to adjust to vaccination suggestions as a result of it requires fewer visits to a clinic, Chin-Hong says.
RSV pictures for pregnant individuals
The respiratory syncytial virus (RSV) is a significant reason for hospitalization amongst infants in the USA. Two methods exist for safeguarding infants from the illness, which inflames the smallest airways of the lungs. Infants can obtain a monoclonal antibody throughout their first eight months, or a pregnant individual can get an RSV vaccine, which in flip protects their toddler (see ‘Born with RSV safety’).
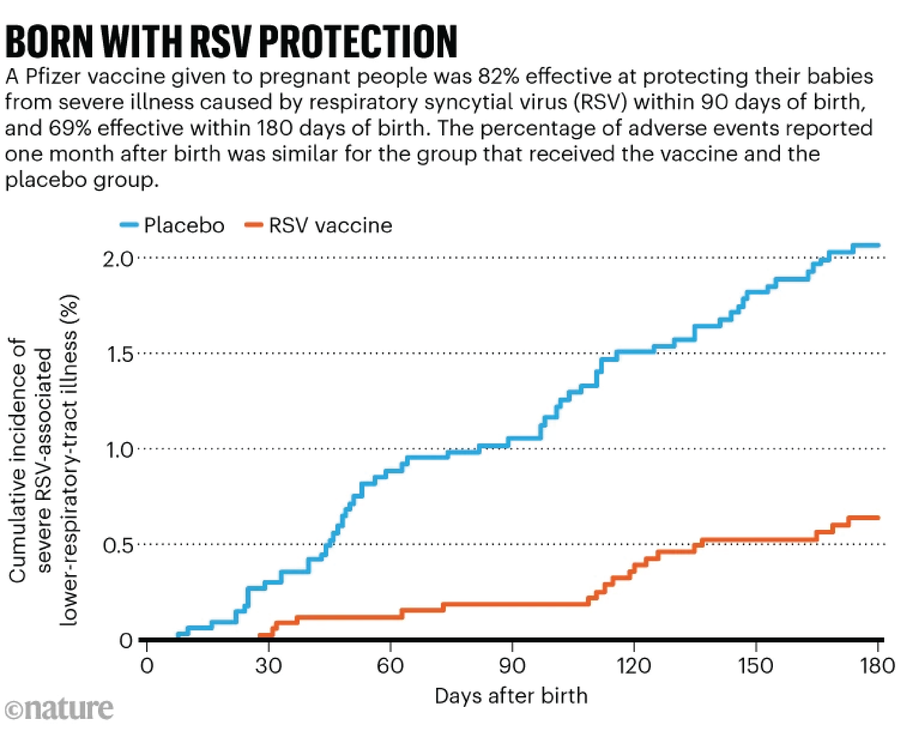
Over the last ACIP assembly, committee members voted to suggest a monoclonal antibody manufactured by pharmaceutical firm Merck for infants underneath eight months not protected against RSV by vaccination throughout gestation. It’s not clear what the committee can be discussing relating to RSV this time, however one doable goal is vaccination of pregnant individuals. At the moment, the CDC recommends that they obtain one dose of an RSV vaccine between 32 and 36 weeks of gestation. In a medical trial of the shot, which is manufactured by pharmaceutical firm Pfizer, there have been barely extra preterm births within the vaccine group than within the placebo group, however the enhance was not statistically vital.
“If we had an ideal system the place each little one acquired the monoclonal antibody preparation, you won’t want maternal vaccinations,” Ratner says. “However in the actual world, that doesn’t at all times occur and there could also be obstacles” for infants to entry the preventive therapy, such because the hospital failing to supply it after start.
This text is reproduced with permission and was first printed on September 12, 2025.

