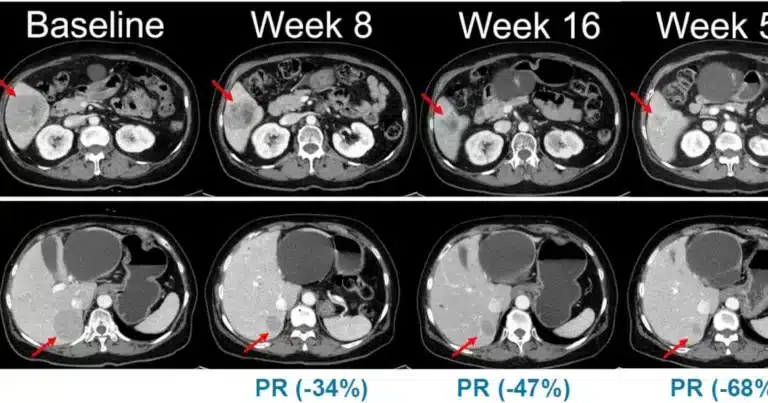
Hanmi Pharm has initiated patient dosing in a Phase 2 clinical trial for its investigational drug Belvarafenib, targeting patients with NRAS-mutated, locally advanced or metastatic melanoma at university hospitals across the country. The first patient received treatment on the 19th.
Drug Approval and Rapid Progress
Belvarafenib secured investigational new drug (IND) approval from the Ministry of Food and Drug Safety’s Drug Safety Evaluation Department in January for this Phase 2 trial. Just one month later, the study advanced to the first patient dosing.
Melanoma and NRAS Mutations
Melanoma represents a challenging cancer with limited treatment options and high risks of recurrence and metastasis. NRAS-mutated cases carry particularly poor prognoses, lacking approved therapies both domestically and internationally.
Currently, Belvarafenib undergoes limited compassionate use, allowing restricted administration to select patients under special approvals.
Mechanism of Action
This oral targeted therapy inhibits RAF and RAS gene mutations within the mitogen-activated protein kinase (MAPK) pathway, which drives tumor cell growth and proliferation.
Trial Overview
The Phase 2 study enrolls 45 patients to assess the efficacy and safety of Belvarafenib combined with the MEK inhibitor cobimetinib.
Hanmi Pharm officials stated, “The Belvarafenib and cobimetinib combination surpasses limitations of prior BRAF monotherapy and BRAF-MEK regimens, offering substantial clinical benefits to broader patient groups with genetic mutations.”