A world staff of researchers led by Duke-NUS has recognized uncommon mutations within the SPNS1 gene as the reason for a beforehand unrecognized multi-organ dysfunction.
A analysis staff led by Duke-NUS Medical Faculty has uncovered the reason for a uncommon, beforehand unidentified illness that impacts a number of organs, offering essential insights and potential pathways for remedy.
Their findings, revealed within the Journal of Scientific Investigation, recognized mutations within the SPNS1 gene as the foundation of the dysfunction, which interferes with how cells recycle fats molecules. The staff confirmed that faulty variations of this gene impair lysosomes (the cell’s recycling facilities), inflicting an unhealthy accumulation of fat and ldl cholesterol that finally results in progressive harm within the liver and muscle mass.
This newly acknowledged situation falls throughout the lysosomal storage illness group, which incorporates greater than 70 uncommon issues linked to failures in mobile recycling.
Tracing the genetic trigger
The breakthrough got here from analyzing two unrelated households whose youngsters skilled unexplained liver illness, muscle weak point, and different signs. Genetic testing revealed mutations in each copies of the SPNS1 gene, which is crucial for transporting broken-down fats molecules out of lysosomes to allow them to be reused all through the cell.
The analysis builds on a earlier Duke-NUS-led examine that pinpointed SPNS1’s position in recycling broken-down fat.
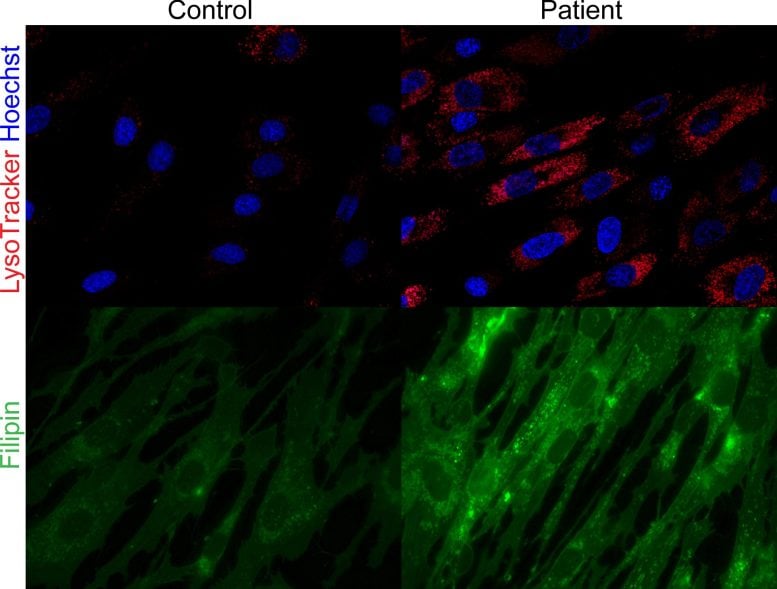
Duke-NUS MD-PhD pupil Ms He Menglan, the examine’s first creator, mentioned that the findings are an important puzzle piece to understanding a illness that remained a thriller for a very long time:
“An vital sort of fats that our mobile recycling programs course of is phospholipids, that are key constructing blocks of cell membranes. In wholesome people, SPNS1 strikes broken-down phospholipids out of lysosomes to be reused to restore membranes or transformed into saved power for the physique. When this intricate course of fails in sufferers with SPNS1 mutations, fats recycling is disrupted, resulting in tissue harm, significantly within the muscle mass and liver.”
Implications for mobile well being
The researchers found that these points turned worse when a key nutrient-sensing system was disrupted, highlighting the significance of SPNS1 in serving to cells reply to nutrient stress and keep power steadiness.
Professor David Silver, Deputy Director of Duke-NUS’ Cardiovascular and Metabolic Issues Programme and senior creator of the examine, mentioned:
“SPNS1 is present in each human cell and performs a key position in recycling phospholipids. Our research revealed that phospholipid recycling by lysosomes performs an important position in regulating how cells keep regular ranges of different vital lipids, reminiscent of fats and ldl cholesterol. These findings open up alternatives to discover the significance of SPNS1 in different ailments reminiscent of most cancers.”
Geared up with these insights, the staff is partnering with N = 1 Collaborative, a corporation growing customized therapies for very uncommon ailments, to translate their findings into bedside options.
Towards customized remedies
Dr Marlen Lauffer, a senior researcher on the Dutch Heart for RNA Therapeutics, Leiden University Medical Center, and a co-author of the study, highlighted the importance of applying these findings in patient care:
“Using what we learned from this research, we are working with the N = 1 Collaborative to create a tailored treatment for the children in our study affected by this condition. This work includes exploring ways to correct the faulty fat transport using new genetic therapies. Our goal is to transform scientific knowledge into therapies that improve the quality of life and give hope to other families facing similar challenges.”
Dr Lauffer added that understanding the precise cause of the disease enables researchers to design treatments that directly target the disrupted pathways, offering options for patients who currently have no treatment path.
Ms Dalila Sabaredzovic, the mother of two of the children in the study, is hopeful that the breakthrough will be the first step towards improving her sons’ quality of life as well as that of others living with the same condition.
“I am so thankful that we now have a foundation to stand on and that work is progressing towards exploring paths of treatments. We feel empowered in many ways we couldn’t before and we really hope that this research will spark not only understanding about the SPNS1 gene and the condition it’s causing, but also a way towards a cure,” she said.
Precision medicine in action
Professor Patrick Tan, Senior Vice-Dean for Research at Duke-NUS, said of the potential of the study’s findings:
“These findings demonstrate the power of precision medicine. By linking unusual patient symptoms to specific genetic mutations, researchers uncover new disease pathways and develop targeted treatments. This approach not only provides answers to families affected by rare diseases but also opens doors for broader medical advances. This discovery is a reminder that even the rarest and most puzzling conditions can be solved—when scientists, clinicians, and families work together.”
This new research reflects Duke-NUS’ commitment efforts to turning scientific discovery into real-world solutions that improve lives.
Reference: “SPNS1 variants cause multiorgan disease and implicate lysophospholipid transport as critical for mTOR-regulated lipid homeostasis” by Menglan He, Mei Ding, Michaela Chocholouskova, Cheen Fei Chin, Martin Engvall, Helena Malmgren, Matias Wagner, Marlen C. Lauffer, Jacob Heisinger, May Christine V. Malicdan, Valerie Allamand, Madeleine Durbeej, Angelica Delgado Vega, Thomas Sejersen, Ann Nordgren, Federico Torta and David L. Silver, 2 September 2025, The Journal of Clinical Investigation.
DOI: 10.1172/JCI193099
Never miss a breakthrough: Join the SciTechDaily newsletter.

